Zhu
Laboratory
 Genetics, Epigenetics and Therapeutic Innovation in Neurodegenerative Diseases
Genetics, Epigenetics and Therapeutic Innovation in Neurodegenerative Diseases
Amyotrophic lateral sclerosis (ALS) is a rare neurodegenerative disease that causes progressive loss of voluntary muscle control and movement, and that leads to total paralysis and death within two to five years of diagnosis. Frontotemporal dementia (FTD) is an umbrella term for a group of neurological disorders clinically characterized by degeneration of the frontal and/or temporal lobes of the brain. FTD patients suffer from personality and behavioral changes, gradual language impairment and other symptoms. FTD is the most common form of dementia for people under age 60. Currently, there is no cure for either ALS or FTD. There can be significant costs for medical care, equipment and home health caregiving later in the disease.
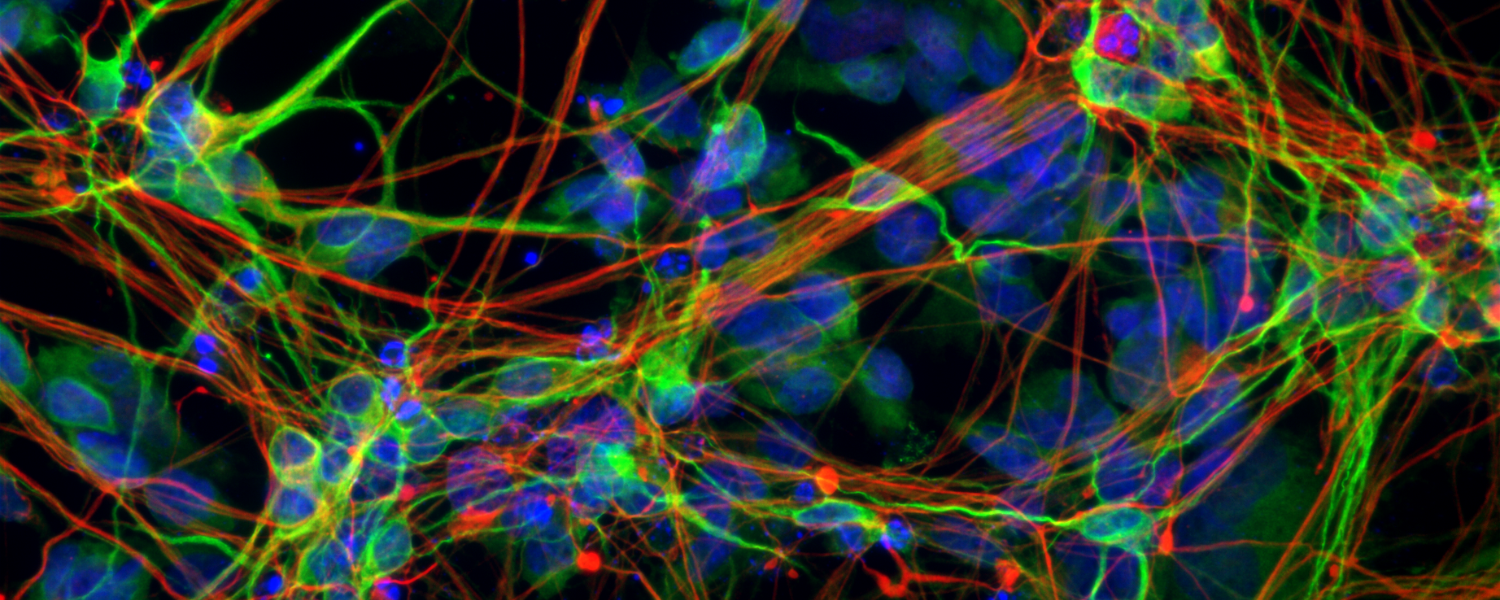
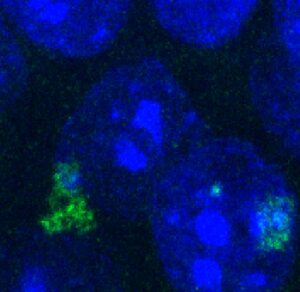
The Zhu Laboratory seeks to reveal the mechanisms that give rise to these devastating diseases and to leverage this knowledge to develop novel strategies for slowing or stopping disease progression. One of the lab’s main research focuses is the large hexanucleotide repeat expansion in the C9ORF72 gene, which is the most common genetic cause for both genetic-inherited familiar ALS and FTD, and also affects a large portion of sporadic cases without family history. Through in-depth studies on this mutation, we can enhance understanding of the mechanistic basis of cognitive and motor activities and answer fundamental questions about motor dysfunction and mental illness, with a particular focus on cell-type specific toxicities, neuron-glia interactions, genetics and epigenetics. To address these challenges, we integrate interdisciplinary approaches including new disease model generation based on genome-modified animals and human iPSCs, CRISPR gene editing, locus-specific epigenetic modification and behavioral neuroscience.
We also will build on previous work by Dr. Zhu, colleagues and biotech industry partners that leads to an antisense oligonucleotide (ASO)-mediated therapy in clinical trials for treating the repeat disease. We expect to discover new drug targets or biomarkers by cutting-edge large-scale screening and single-cell multiomics and develop therapies for neurological disorders through ASO, small-molecule drug, AAV-mediated gene therapy or other novel methods.
Join Our Team
Dr. Qiang Zhu’s Lab is seeking a highly motivated Postdoctoral Fellow to join our team. Learn more and apply today!
Our Impact
We’re raising thousands to save millions.
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 122 peer-reviewed papers published in 2024, 63 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials co-funded by VAI & Cure Parkinson's (out of 41 total International Linked Clinical Trials Program trials)
Qiang Zhu, Ph.D.
Assistant Professor, Department of Neurodegenerative Science
Areas of Expertise
Amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), neuron-glia interactions, genetics, epigenetics, behavioral neuroscience, C9ORF72 repeat expansion, SOD1, neurodegenerative disease models, antisense oligonucleotides
Biography
Qiang Zhu, Ph.D., is a neuroscientist with deep expertise in the molecular and cellular mechanisms that give rise to neurodegenerative diseases. He earned his Ph.D. in anatomical sciences and neurobiology from the University of Louisville School of Medicine. He then joined the lab of Dr. Don Cleveland at University of California, San Diego, as a postdoctoral fellow. While there, he co-led a collaborative team of researchers from academia and industry to investigate the C9ORF72 repeat expansion, the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). His work established the feasibility of an antisense oligonucleotide (ASO)-mediated therapy, which is currently in clinical trials for treating the C9ORF72 ALS/FTD. He further elucidated the synergistic effects of gain of repeat toxicity and loss of the C9ORF72 function in driving the C9ORF72-associated ALS and FTD, which revealed new opportunities for drug development targeting this mutation. He recently developed new motor neuron disease models and is collaborating with the biotech industry to explore new treatments. Dr. Zhu has earned several awards for his scholarship, including the Milton Safenowitz Postdoctoral Fellowship and the Starter Grant from the ALS Association.
Patent
Methods for reducing c9orf72 expression. PCT/US2017/027355, US2019/0142856 A1, 2016
Selected Publications
* denotes equal contribution
Zhu Q*, Jiang J*, Gendron TF, McAlonis-Downes M, Jiang L, Taylor A, Diaz Garcia S, Dastidar GS, Rodriguez M, King P, Zhang YJ, Spada A, Xu H, Petrucelli L, Ravits J, Da Cruz S, Lagier-Tourenne C, Cleveland DW. 2020. Reduced C9ORF72 function exacerbates gain-of-toxicity from ALS/FTD-causing repeat expansions in C9orf72. Nat Neurosci 23(5):615–624.
Highlighted by News & Views. Lutz C. 2020. Realizing the gains and losses in C9ORF72 ALS/FTD. Nat Neurosci. 23(5):596–597.
Xiao G, Du J, Wu h, Ge X, Xu X, Yang A, Zhu Y, Hu X, Zheng K, Zhu Q, Qiu M. 2020. Differential inhibition of Sox10 functions by Notch-Hes pathway. Cell Mol Neurobiol 40(4):653–662.
Jiang J*, Zhu Q*, Gendron TF*, Saberi S, McAlonis-Downes M, Seelman A, Stauffer JE, Jafar-Nejad P, Drenner K, Schulte D, Chun S, Sun S, Ling SC, Myers B, Engelhardt J, Katz M, Baughn M, Platoshyn O, Marsala M, Watt A, Heyser CJ, Ard MC, De Muynck L, Daughrity LM, Swing DA, Tessarollo L, Jung CJ, Delpoux A, Utzschneider DT, Hedrick SM, de Jong PJ, Edbauer D, Van Damme P, Petrucelli L, Shaw CE, Bennett CF, Da Cruz S, Ravits J, Rigo F, Cleveland DW, Lagier-Tourenne C. 2016. Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron 90(3):535–550.
Commented by Preview. Hayes LR, Rothstein JD. 2016. C9ORF72-ALS/FTD: Transgenic mice make a come-BAC. Neuron. 90(3):427–431.
Zhu Q, Tan Z, Zhao SF, Huang H, Zhao XF, Hu XM, Zhang YP, Shields CB, Uetani N and Qiu M. 2015. Developmental expression and function of protein tyrosine phosphatase receptor type D in oligodendrocyte myelination. Neuroscience 308:106–114.
Zhu Q*, Zhao X*, Zheng K*, Li H, Huang H, Zhang Z, Mastracci T, Wegner M, Chen Y, Sussel L, Qiu M. 2014. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the developing CNS. Development 141(3):548–555.
Zhao X*, Chen Y*, Zhu Q, Huang H, Teng P, Zheng K, Hu X, Xie B, Zhang Z, Sander M, Qiu M. 2014. Control of astrocyte progenitor specification, migration and maturation by Nkx6.1 homeodomain transcription factor. PLoS One 9(10):e109171.
Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, Jiang J, Watt AT, Chun S, Katz M, Qiu J, Sun Y, Ling SC, Zhu Q, Polymenidou M, Drenner K, Artates JW, McAlonis-Downes M, Markmiller S, Hutt KR, Pizzo DP, Cady J, Harms MB, Baloh RH, Vandenberg SR, Yeo GW, Fu XD, Bennett CF, Cleveland DW, Ravits J. 2013. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A 110(47):E4530–4539.
Zhu Q, Whittemore SR, DeVries WH, Zhao XF, Kuypers NJ, Qiu M. 2011. Dorsally derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia 59(11):1612–1621.
Cai J, Zhu Q, Zheng K, Li H, Cao QL and Qiu M. 2010. Co-localization of Nkx6.2 and Nkx2.2 homeodomain proteins in differentiated myelinating oligodendrocytes. Glia 58(4):458–468.
Zheng K, Li H, Zhu Y, Zhu Q and Qiu M. 2010. MicroRNAs are essential for the developmental switch from neurogenesis to gliogenesis in the developing spinal cord. J Neurosci 30(24):8245–8250.
Park J, Liu B, Chen T, Li H, Hu, X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J, Peng X and Qiu M. 2008. Disruption of Necl-1 cell adhesion molecule leads to delayed axonal myelination in the developing nervous system. J Neurosci 28 (48):12815–12819.
Zhao S, Hu X, Park J, Zhu Y, Zhu Q, Luo C, Han R, Cooper N. and Qiu M. 2007. Selective expression of LDLR and VLDLR in myelinating oligodendrocytes. Develop Dynam 236 (9):2708–2712.
Zhu Q, Chang H, Chen Y, Fang F, Xue C, Zhang F, Qiu M, Wang H, Wang B, Chen Z. 2005. Protection of inactivated influenza virus vaccine against lethal influenza virus infection in diabetic mice. Biochem Biophys Res Commun 329(1):87c94.
Tang L, Zhu Q, Qin E, Yu M, Ding Z, Shi H, Cheng X, Wang C, Chang G, Zhu Q, Fang F, Chang H, Li S, Zhang X, Chen X, Yu J, Wang J, Chen Z. 2004. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol 23(6):391–394.


Diamante Balcazar
Assistant Research Technician, Department of Neurodegenerative Science

Ashley Douglass, B.S.
Senior Administrative Assistant II

Yingli Gu M.D., Ph.D.
Research Associate, Department of Neurodegenerative Science


Laura Pascoe
Assistant Research Technician, Department of Neurodegenerative Science

Zhongliang Zou
Research Technician, Department of Neurodegenerative Science

 Genetics, Epigenetics and Therapeutic Innovation in Neurodegenerative Diseases
Genetics, Epigenetics and Therapeutic Innovation in Neurodegenerative Diseases